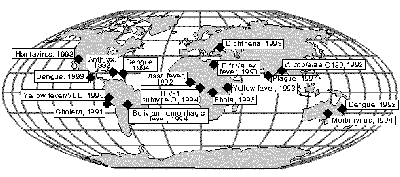
Global Microbial Threats in the 1990s

For the U.S. Government to help in controlling an incipient - or raging - epidemic in another country, three things must occur. First, reliable information must reach the United States. Second, U.S. scientists and public health officials must evaluate the information and decide what measures should be taken. Third, U.S. officials must help the affected country implement those measures. However, U.S. participation in an epidemiologic investigation within another nation is dependent upon a formal request for assistance from the foreign government. This was the pattern of events during the Ebola virus investigation (see "Lessons Learned From the Ebola Virus Outbreak in Zaire"). If no request is received, our Government may still take action to minimize the risk of disease importation into the United States (see "Plague in India").
In some cases -- if the notification arrives quickly enough -- this informal surveillance system works. When international resources are successfully mobilized, assistance in diagnosis, disease control and prevention can be made available to local health authorities. Clinical specimens can be sent to a diagnostic reference laboratory to rule out known disease agents (see "The Informal Global Network"). Epidemiologists can be sent into the field to investigate the source of the new infection and determine how it is transmitted. Public health officials can use this information to implement appropriate control measures. Once the infectious agent has been identified, which is often a difficult task, experimental scientists can start to develop diagnostic tools and treatments if the agent is a newly recognized one.
However, a new infectious disease can be easily overlooked, especially when the disease originates in a part of the world that lacks effective domestic disease surveillance and modern communications. Left unchecked, the disease may spread far and wide before it is recognized and reported.
Lack of an executive function for response to epidemics. The U.S.Government response to international epidemics occurs on an ad hoc basis. As described below (and in the inventory that accompanies this report), many Government agencies and departments have resources that can facilitate an effective response to epidemics of infectious diseases.
The authority of CDC, for instance, does not cover international disease control and prevention, and USAID has limited technical and financial resources in this area. In practice, individual Government workers who become aware of outbreaks do what they can to coordinate agency efforts and provide aid to affected countries. But there is no formal structure or designated resources for this activity.
Resources for emergency responses. At present, the U.S. Government has no funds set aside for responses to international disease outbreaks. Government disaster assistance groups such as the Federal Emergency Management Administration and USAID's Office of Foreign Disaster Assistance (OFDA) do not take responsibility for infectious disease emergencies. At CDC although approximately 65% of the budget is dedicated to the prevention and control of infectious diseases, about 95% of these funds are earmarked for AIDS, TB, and sexually transmitted diseases and vaccine preventable diseases. Moreover, USAID has limited resources available for international outbreak investigations. Thus, when a new or re-emerging disease is suspected in another country, there is very little flexibility in any U.S. Government agency's budget to provide for an investigation.
Importation of infectious diseases into the United States. Each time an infected person (or a contaminated food or sick animal) enters the United States, an opportunity arises for a contagious microbe to spread to the American people. CDC strives to prevent this in two ways. One protective measure is to issue advisories that caution against travel to or from the site of an epidemic. CDC also provides information on travelers' health, including information on recommended vaccinations and on regimens for drug prophylaxis. A more comprehensive line of defense relies on local surveillance systems, at the state, county, and city levels. Unfortunately, our local public health surveillance systems are no longer adequate because of our past complacency about infectious diseases, poor planning, and lack of resources.
Screening of travelers at U.S. ports of entry. Under the Public Health Service Act and the Foreign Quarantine Regulations, all aircraft and ships captains are required to radio the nearest CDC quarantine station at their port of arrival when they have an ill person or when a passenger has died. CDC has the authority to detain, isolate, or conditionally release any person believed to be infected or exposed to a communicable disease. CDC staffs quarantine stations at seven ports of entry at major airports in New York, Miami, Chicago, Seattle, San Francisco, Los Angeles, and Honolulu. Each station provides backup for other ports in their geographic area of responsibility. At ports of entry where CDC does not have staff, the gap is filled by airline workers, by physicians on contract with CDC, and by officials of the Immigration and Naturalization Service (INS). U.S. civilians, foreign nationals (including tourists, business travelers, long-term visitors), and immigrants can enter at any of these airports, as well as seaports and land border areas. There are approximately 50 international airports in the United States and more than 150 other legal entry points.
The identification of persons carrying pathogens capable of causing serious disease outbreaks is made difficult by the very large number of people entering the United States from increasingly remote locations. Most American cities can be reached within 36 hours from anywhere in the world, either by direct or by connecting flights. The incubation periods of most infectious diseases (the time between infection and the appearance of symptoms) is considerably longer than 36 hours. Because only obviously ill patients are identified by screening at ports of entry, routine state and local surveillance efforts are relied on to identify infected travelers who become ill some time after entry into the United States.
Screening of soldiers. Military personnel who return to the United States are not routinely quarantined. Military personnel who become ill overseas are evacuated to DoD medical facilities in the United States. Military personnel who are not sick return to their unit bases. Deployed reservists are more apt to re-enter civilian health-care channels than active duty personnel. The medical tracking of all deployed military personnel after they return home is being improved by DoD to facilitate the recognition and diagnosis of latent infections.
Food-borne and animal-borne diseases. CDC's quarantine program also coordinates with the U.S. Department of Agriculture (USDA), U.S. Fish and Wildlife Services, Department of Interior, and FDA to ensure that other possible carriers of human disease (food and animals) are managed appropriately.
USDA's Food Safety and Inspection Service (FSIS) plays an important role in disease control and eradication. FSIS samples food products for a number of pathogens and protects the food supply by retaining or recalling products. FSIS inspects for conditions and collects samples to test for many diseases such as rabies, tuberculosis, brucellosis, and pseudorabies which can be transmitted to humans. This inspection is crucial for the surveillance and monitoring system of the USDA-APHIS.
The Animal and Plant Health Inspection Service (APHIS) of the
USDA is responsible for protecting American livestock and poultry
from foreign and domestic diseases. Many diseases of humans are
carried by and transmitted from animals or animal products
(Ebola, anthrax, cryptosporidium, hantavirus, Rift Valley fever,
Lyme disease, E. coli, tuberculosis, brucellosis, rabies,
pseudorabies, to name to few). APHIS carries out this
responsibility through several activities:
The USDA's animal health infrastructure and mission is, in part, built on the important task of excluding and rapidly responding to the introduction of these pests and diseases. APHIS inspects animals entering the United States from foreign countries at the border or port of entry. APHIS establishes quarantine and testing requirements for imported animals to reduce the risk of diseases and operates several USDA quarantine facilities.
In addition to exclusion activities, APHIS operates programs to control and eliminate diseases in domestic livestock, including those that also affect humans. Interstate movement and transport of infected and exposed animals are regulated in an effort to stop further spread of the diseases. Monitoring of animal diseases is maintained through APHIS' National Animal Health Monitoring System.
Successful Surveillance to Prevent Disease Transmission:
Venezuelan Equine Encephalitis in Peru
During 1994 and early 1995, the U. S. Naval Medical Research
Institute Detachment (NAMRID) in Lima, Peru, detected several
cases of dengue fever, oropouche, and Venezuelan equine
encephalitis (VEE) in northern Peru. These diseases are caused by
arboviruses, which are carried by insect vectors, and vaccines
against several arboviral illnesses are available. CDC followed
up on the NAMRID reports and determined that VEE had occurred
among Peruvian soldiers stationed in the area of the border
dispute with Ecuador. The health authorities in Peru and Ecuador
were notified and control measures were implemented.
After these occurrences, it came to the attention of CDC that the U.S. Army was planning to deploy troops in this area to mediate the border dispute. CDC notified the U.S. Army at Fort Detrick, Maryland, and the Southern Command in Panama, and advised that all troops be immunized against VEE before deployment.
An Epidemic Spreads from Continent to Continent: Dengue Fever in
Asia
In recent years several Caribbean countries have experienced
epidemics of dengue fever but have failed to report them, fearing
that the news would have a negative impact on their tourist
industries. The outbreaks became known only after tourists
returning to their home countries became ill.
Although CDC and WHO received rumors of outbreaks of dengue and dengue hemorrhagic fever (DHF) in Asia during the late 1980s, CDC did not receive official information about them, and no diagnostic samples were sent for confirmation. (DHF and dengue fever are different clinical manifestations of the same viral infection.) Eventually, CDC's WHO Collaborating Centre for Reference and Research on Dengue and DHF received blood samples from a pediatrician in the area of Asia, and the presence of a specific strain of dengue virus was confirmed. In 1994, when dengue fever broke out in Central America, scientists isolated the same strain of virus from the Central American blood samples, indicating that the virus that caused DHF in Asia had spread to the Americas.
The U.S. Government took several steps to ensure that plague would not be imported into the United States. The State Department invited two American epidemiologists to New Delhi to assist the U.S. embassy and to be available if Indian doctors or political authorities requested help. In addition, CDC issued advisories to international travelers, notified state health authorities, and increased surveillance at U.S. airports. FDA worked with pharmaceutical manufacturers to accelerate efforts to increase supplies of plague vaccine. In October, CDC participated in a WHO-led investigation, and by October 27 determined that no infectious disease emergency existed. Effective surveillance, followed by prompt diagnosis and treatment, could have reduced the magnitude of the crisis and saved India much of the estimated $2 billion in revenues lost from tourism, exports, and shipping. The U.S. agencies which participated in the Government response to the plague in India included the Departments of State, Justice (Immigration and Naturalization Service), Agriculture, and Transportation; the Public Health Service (including CDC and FDA) of the Department of Health and Human Services; USAID; and state and local health departments. Despite the cooperation of these agencies, the U.S. Government had domestic obstacles to overcome in responding to this international health emergency. At present, CDC has the only laboratory in the world that serves as a reference laboratory for plague. Unfortunately, support for that laboratory has decreased to the point where there is only one full-time employee with experience and training in plague epidemiology and treatment. To respond effectively, CDC had to pull staff and resources from other programs.
Sentinel Surveillance System: A network of individuals, facilities, or laboratories that monitors changes in the incidence of disease in a systematic way. Such networks usually include many strategically located outposts and are designed to serve as early warning systems for disease outbreaks.
Epidemic or Outbreak: The occurrence of cases of a disease above the expected number or baseline level, usually over a given period of time, in a geographic area, or in a specific population group.
Emerging Infection: A new or newly identified pathogen or syndrome which has been recognized over the last two decades, or which has resulted in new manifestations of disease.
Re-emerging Infection: A known or previously identified pathogen or syndrome which is increasing in incidence, expanding into new geographic areas, affecting new population groups, or which threatens to increase in the near future.
Zoonosis: A disease that can be transmitted from animals to humans.
 Return to CISET Report Home Page
Return to CISET Report Home Page